COVID-19 or Flu?
Get the answers with Flowflex PLUS
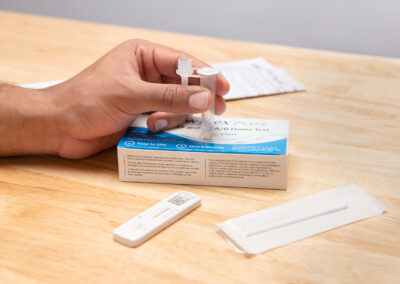
The Flowflex® PLUS COVID-19 and Flu A/B Home Test is all you need to determine your family’s COVID-19/Flu status.
Symptoms may be similar, treatments for complications differ
- 3-in-1 test for COVID-19, Flu A, and Flu B
- Easy-to-use nasal swab test
- Can be used to test children as young as 2 years old
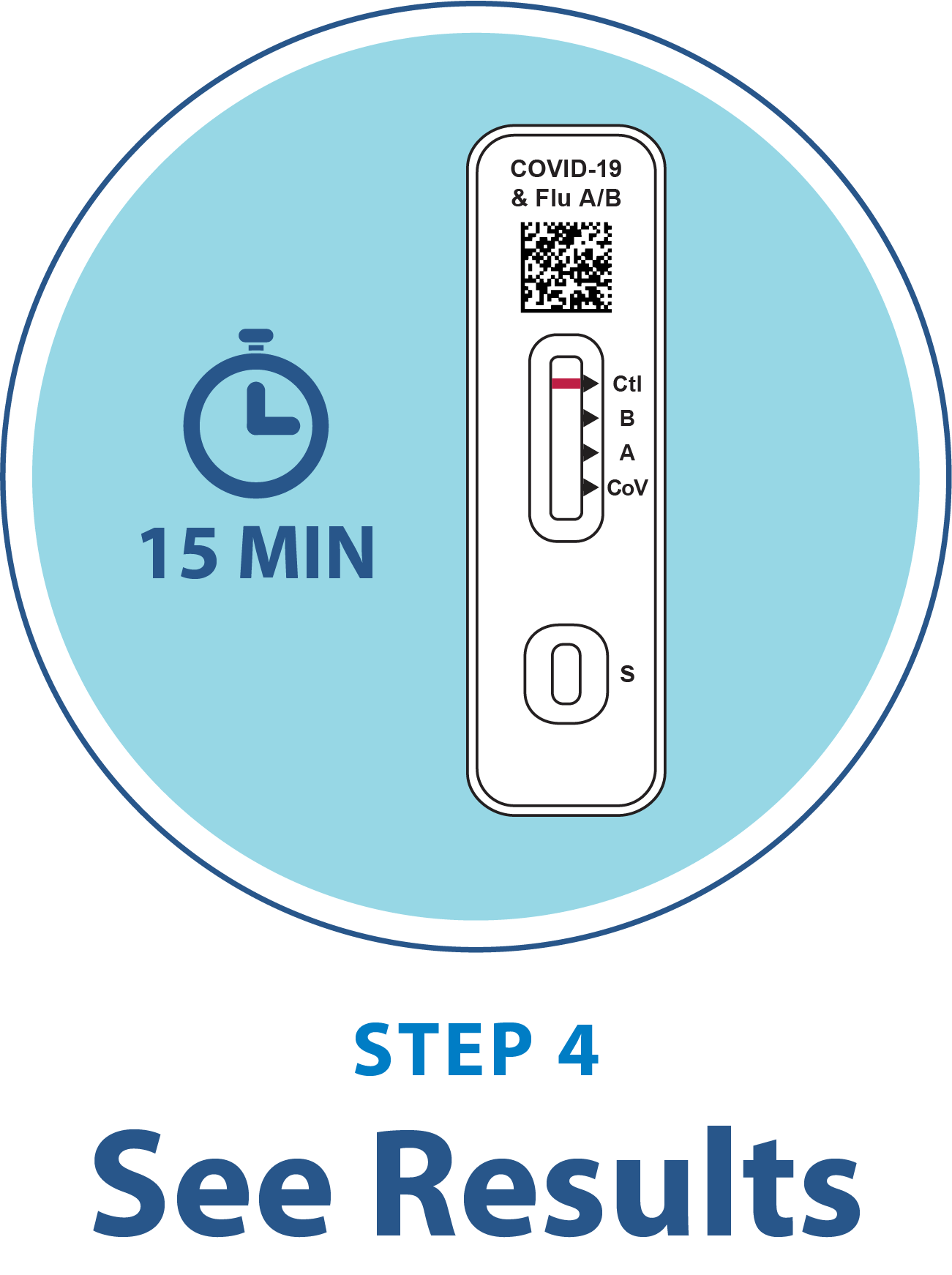
- Results in 15 minutes
- No need to send off to a lab to obtain results
- Medical-grade technology used in doctor’s offices
- Compact packaging for “On-The-Go” testing
![[3D]1130671601-1T The Flowflex™ COVID-19 Antigen Home Test](https://flowflexcovid.com/wp-content/uploads/2024/07/3D1130671601-1T.png)
Expiration Date Extension
As of April 4, 2025, the expiration date of the Flowflex Plus COVID-19 and Flu A/B Home Test has been extended by 6 months. Please click here to check the new expiration date using the lot number printed on the test kit box.
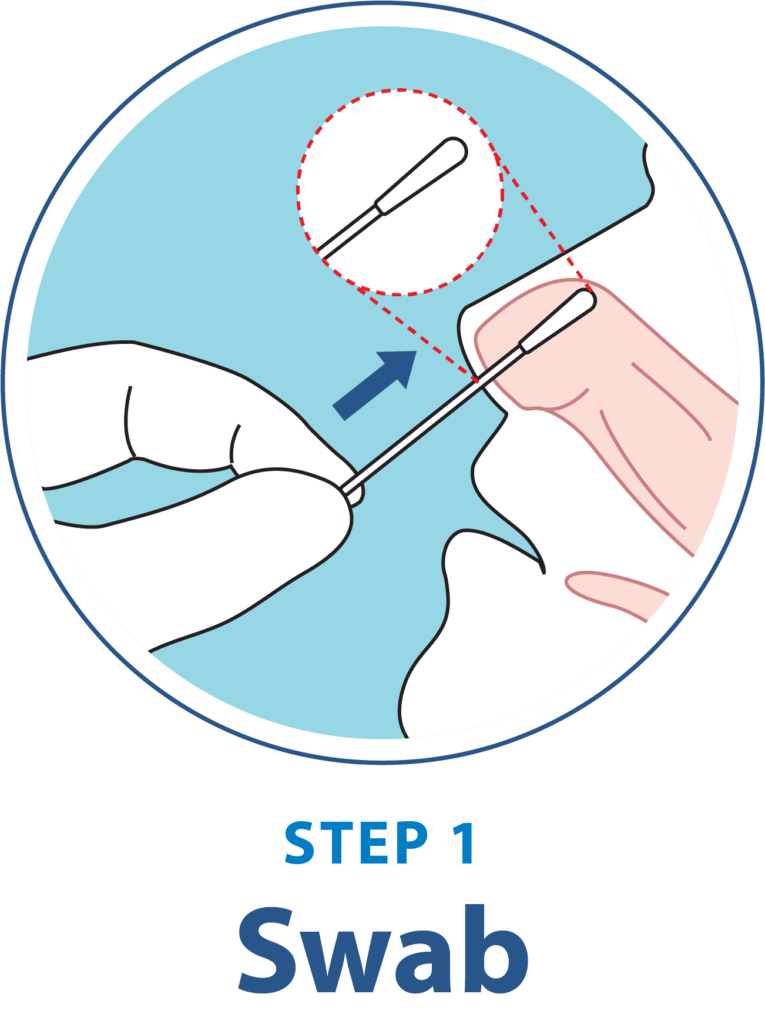
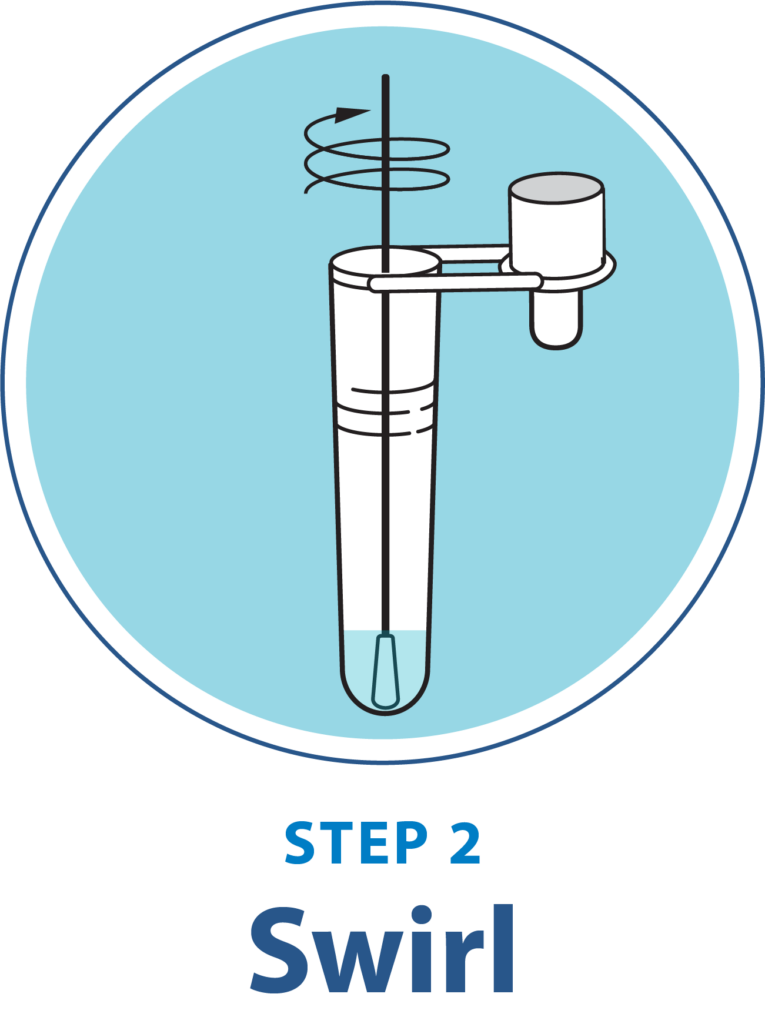
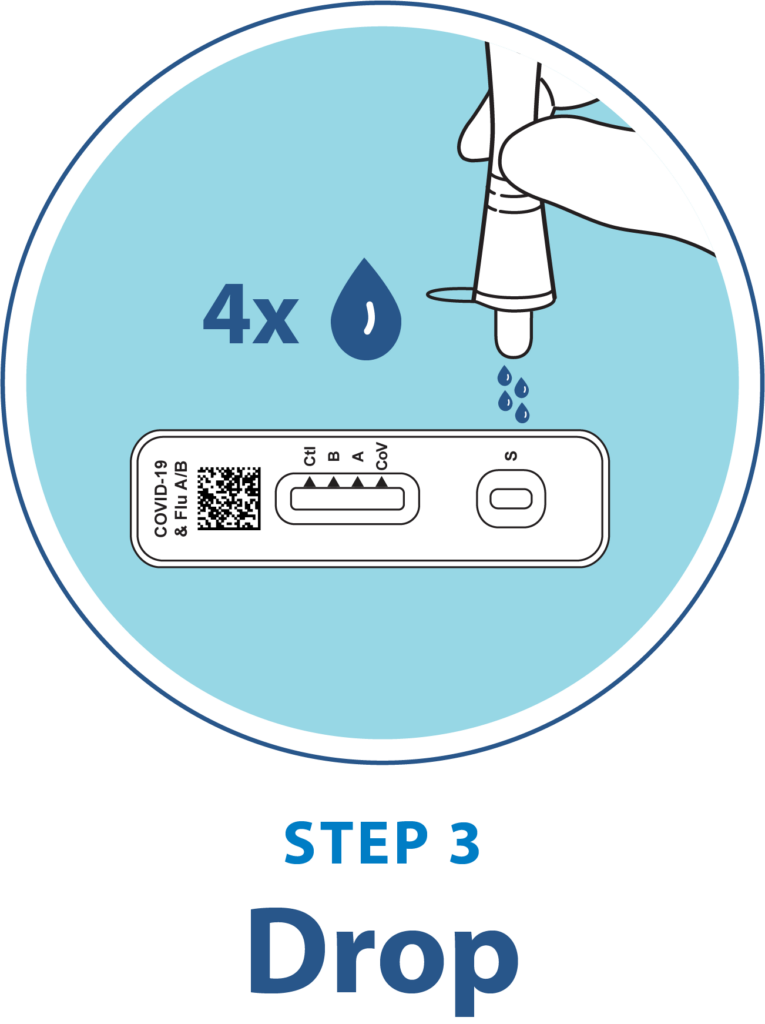
Test Procedure Overview
Resources
Quick Reference Instructions (English)
Quick Reference Instructions (Spanish)
Fact Sheet for Healthcare Professionals
Healthcare Provider Package Insert
Quick Instruction Card (English)
Quick Instruction Card (Spanish)
Frequently Asked Questions
Need Support?
News
Flowflex® Plus COVID-19 + Flu A/B Home Test Receives FDA 510(k) Clearance
SAN DIEGO, CA., May 12, 2025 – ACON Laboratories, Inc., a leading global medical device manufacturer, announced today that its Flowflex® Plus COVID-19 + Flu A/B Home Test has received 510(k) clearance from the U.S. Food & Drug Administration (FDA)....
Flowflex Plus COVID-19 and Flu A/B Home Test New Shelf-life Extension
Since the launch of its Flowflex Plus COVID-19 and Flu A/B Home Test, ACON Laboratories, Inc. has continued testing for product stability to extend the shelf-life. These results have been shared with the FDA. We are pleased to announce that the request to extend...
Flowflex® Plus COVID-19 and Flu A/B Home Test Receives FDA EUA
SAN DIEGO, CA., July 29, 2024 – ACON Laboratories, Inc., a leading global medical device manufacturer for 25 years, announced today that its Flowflex® Plus COVID-19 and Flu A/B Home Test has been authorized for emergency use by the U.S. Food and Drug Administration....
- In the USA, this product has not been FDA cleared or approved, but has been authorized by FDA under an Emergency Use Authorization.
- This product has been authorized only for the detection of proteins from SARS-CoV-2, influenza A and influenza B, not for any other viruses or pathogens.
- The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. §360bbb- 3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
For more information on EUAs please visit: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization
For the most up to date information on COVID-19, please visit: www.cdc.gov/COVID19




![[3D]1130671901-25T The Flowflex™ COVID-19 Antigen Home Test](https://flowflexcovid.com/wp-content/uploads/2024/07/3D1130671901-25T.png)

