Get Back to Sharing Special Moments
with Family and Friends
Find peace of mind with Flowflex!
- Easy-to-use nasal swab test
- Can be used to test children as young as 2 years old
- Results in 15 minutes
- No need to send off to a lab to obtain results
- Compact packaging for “On-The-Go” testing

Expiration Date Extension
All the Flowflex COVID-19 Antigen Home Test lot numbers eligible for the expiration date extension have expired. Please follow the expiration dates printed on the kit box and cassette pouch.
Please click here for the Flowflex COVID-19 Antigen Home Test (EUA210494)
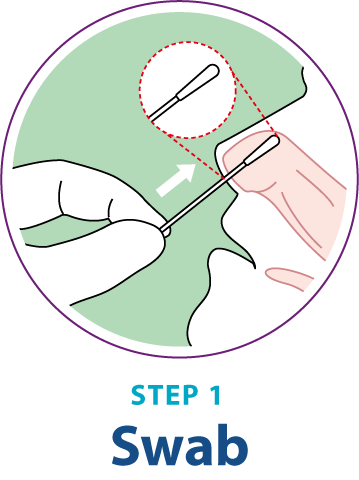
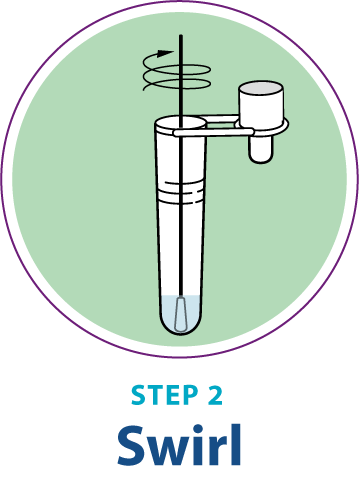
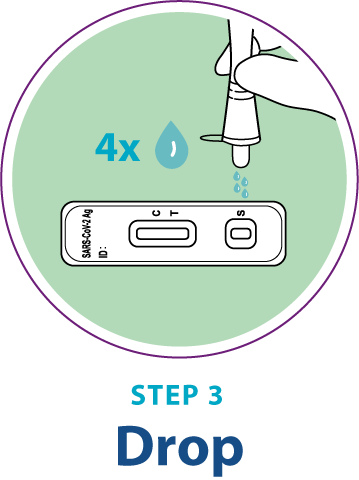
Test Procedure Overview
This test procedure overview does not replace the package insert. Before you begin the test, it is important to read and follow the detailed instructions in the package insert.
Resources
Consumer Package Insert (English)
Consumer Package Insert (Spanish)
Healthcare Provider Package Insert
Quick Reference Instructions (English)
Quick Reference Instructions (Spanish)
Frequently Asked Questions
Where Can I Buy It?
For employee testing solutions, click here.
Need Support?
News
STATEMENT ON THE OMICRON BA.2.86 and JN.1 VARIANTS
ACON Laboratories, Inc. has been continuously monitoring the emergence of SARS-CoV-2 variants. According to the Centers for Disease Control and Prevention (CDC) COVID Data Tracker, the Omicron variant remains the dominant variant circulating in the United States....
ACON Receives FDA 510(k) Clearance for Flowflex® COVID-19 Test; Will be Manufactured in San Diego
SAN DIEGO, CA, November 9, 2023 – ACON Laboratories, Inc. is proud to announce that the U.S. Food & Drug Administration (FDA) has granted 510(k) marketing clearance for the Flowflex® COVID-19 Antigen Home Test. This is the first FDA 510(k) for an over-the-counter...
Recall Issued by the Philadelphia Department of Public Health for Counterfeit Flowflex COVID-19 Tests
ACON Laboratories, Inc. is aware of the announcement by the Philadelphia Department of Public Health of a recall of certain at-home COVID-19 tests on September 9, 2023, and actively working with them and the FDA to conduct further investigation. The Lot number of...