NEW
Flowflex® COVID-19
Antigen Home Test
A Simple Way to Test for COVID-19
Get Back to Sharing Special Moments
with Family and Friends
Find peace of mind with Flowflex!
- Easy-to-use nasal swab test
- Can be used to test children as young as 2 years old
- For use with and without COVID-19 symptoms
- Results in 15 minutes
- No need to send off to a lab to obtain results
- Compact packaging for “On-The-Go” testing
![[3D]1130525701-w-FSA The Flowflex™ COVID-19 Antigen Home Test](https://flowflexcovid.com/wp-content/uploads/2024/01/flowflex_1t-box_fsa-hsa.png)
Expiration Date Extension
All the Flowflex COVID-19 Antigen Home Test lot numbers eligible for the expiration date extension have expired. Please follow the expiration dates printed on the kit box and cassette pouch.
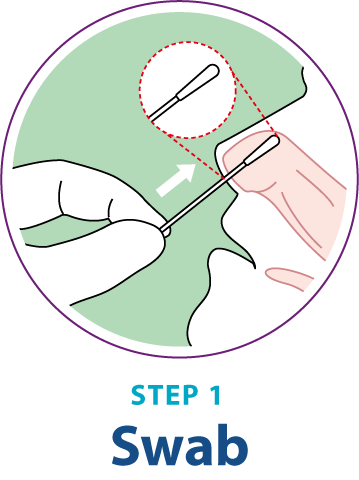
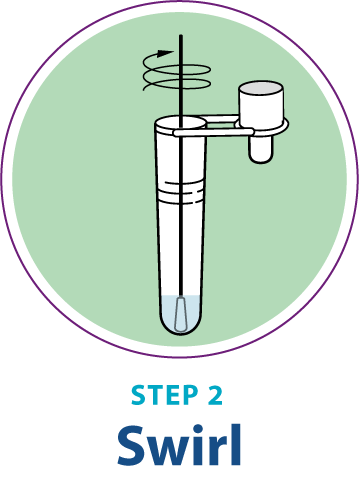
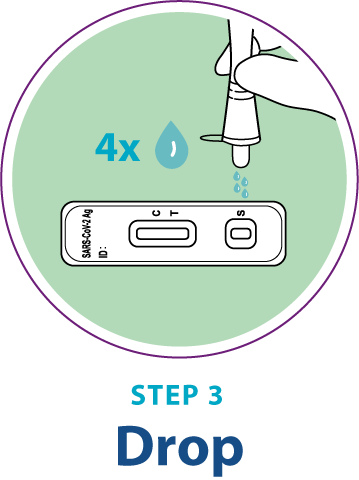
Test Procedure Overview
This test procedure overview does not replace the package insert. Before you begin the test, it is important to read and follow the detailed instructions in the package insert.
Resources
Consumer Package Insert (English)
Consumer Package Insert (Spanish)
Fact Sheet for Healthcare Professionals
Healthcare Provider Package Insert
Quick Reference Instructions (English)
Quick Reference Instructions (Spanish)
Frequently Asked Questions
Where Can I Buy It?
For employee testing solutions, click here.
Need Support?
News
STATEMENT ON THE OMICRON BA.2.86 and JN.1 VARIANTS
ACON Laboratories, Inc. has been continuously monitoring the emergence of SARS-CoV-2 variants. According to the Centers for Disease Control and Prevention (CDC) COVID Data Tracker, the Omicron variant remains the dominant variant circulating in the United States....
ACON Receives FDA 510(k) Clearance for Flowflex® COVID-19 Test; Will be Manufactured in San Diego
SAN DIEGO, CA, November 9, 2023 – ACON Laboratories, Inc. is proud to announce that the U.S. Food & Drug Administration (FDA) has granted 510(k) marketing clearance for the Flowflex® COVID-19 Antigen Home Test. This is the first FDA 510(k) for an over-the-counter...
Recall Issued by the Philadelphia Department of Public Health for Counterfeit Flowflex COVID-19 Tests
ACON Laboratories, Inc. is aware of the announcement by the Philadelphia Department of Public Health of a recall of certain at-home COVID-19 tests on September 9, 2023, and actively working with them and the FDA to conduct further investigation. The Lot number of...
- In the USA, this product has not been FDA cleared or approved; but has been authorized by FDA under an EUA;
- This product has been authorized only for the detection of proteins from SARS- CoV-2, not for any other viruses or pathogens; and,
- The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
For more information on EUAs please visit: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization
For the most up to date information on COVID-19, please visit: www.cdc.gov/COVID19














![[3D]1130553301@2x The Flowflex™ COVID-19 Antigen Home Test](https://flowflexcovid.com/wp-content/uploads/2024/01/flowflex_25t-box_fsa-hsa.png)

