NEW
Flowflex® COVID-19
Antigen Home Test
A Simple Way to Test for COVID-19
Get Back to Sharing Special Moments
with Family and Friends
Find peace of mind with Flowflex!
- Easy-to-use nasal swab test
- Can be used to test children as young as 2 years old
- For use with and without COVID-19 symptoms
- Results in 15 minutes
- No need to send off to a lab to obtain results
- Compact packaging for “On-The-Go” testing
![[3D]1130525701-w-FSA The Flowflex™ COVID-19 Antigen Home Test](https://flowflexcovid.com/wp-content/uploads/2024/01/flowflex_1t-box_fsa-hsa.png)
Expiration Date Extension
All the Flowflex COVID-19 Antigen Home Test lot numbers eligible for the expiration date extension have expired. Please follow the expiration dates printed on the kit box and cassette pouch.
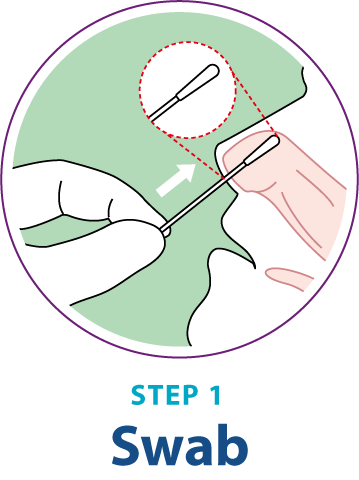
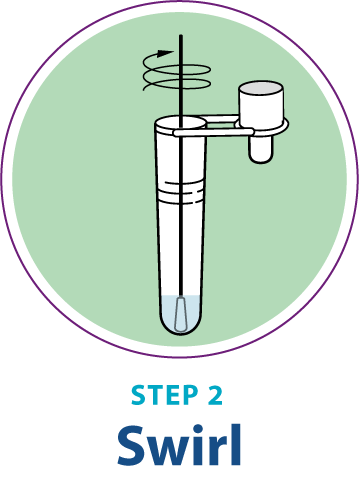
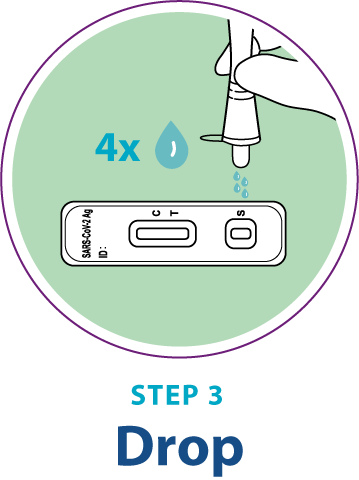
Test Procedure Overview
This test procedure overview does not replace the package insert. Before you begin the test, it is important to read and follow the detailed instructions in the package insert.
Resources
Consumer Package Insert (English)
Consumer Package Insert (Spanish)
Fact Sheet for Healthcare Professionals
Healthcare Provider Package Insert
Quick Reference Instructions (English)
Quick Reference Instructions (Spanish)
Frequently Asked Questions
Where Can I Buy It?
For employee testing solutions, click here.
Need Support?
News
ACON Laboratories Issues a Recall of non-EUA Authorized “Flowflex™ SARS-CoV-2 Antigen Rapid Test (Self-Testing)” Tests From the U.S. Market
SAN DIEGO, CA, January 9, 2022 – ACON Laboratories, Inc. (“ACON Laboratories”), the legal manufacturer of the “Flowflex™ COVID-19 Antigen Home Test” (FDA Emergency Use Authorization EUA210494), has identified the U.S. distribution of unauthorized, adulterated and...
UPDATED STATEMENT ON THE OMICRON VARIANT
ACON Laboratories, Inc. has been continuously monitoring the emergence of SARS-CoV-2 variants, including the Omicron variant. We are pleased to report that an independent evaluation conducted by the National Institutes of Health’s (NIH) RADx program has indicated that...
STATEMENT ON THE OMICRON VARIANT
We at ACON Laboratories, Inc. have been continuously monitoring the emergence of SARS-CoV-2 variants and the Omicron variant is no exception. The Omicron variant is notable for the significant number of mutations in the Spike protein. Those mutations are not relevant...
- In the USA, this product has not been FDA cleared or approved; but has been authorized by FDA under an EUA;
- This product has been authorized only for the detection of proteins from SARS- CoV-2, not for any other viruses or pathogens; and,
- The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
For more information on EUAs please visit: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization
For the most up to date information on COVID-19, please visit: www.cdc.gov/COVID19














![[3D]1130553301@2x The Flowflex™ COVID-19 Antigen Home Test](https://flowflexcovid.com/wp-content/uploads/2024/01/flowflex_25t-box_fsa-hsa.png)

