Flu or COVID?
One Test.
Get the trusted home test.
One Test. 15 Min.
*Source: Circana Retail Sales Data (Units sold)
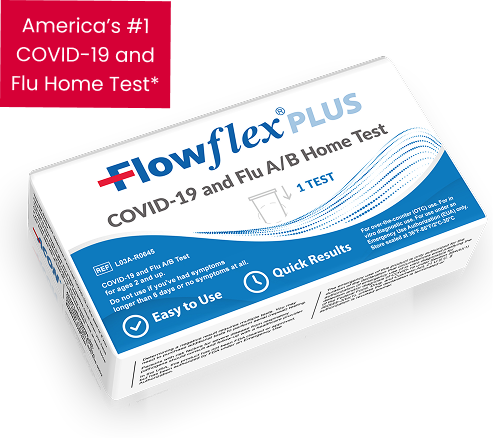
Flowflex Plus COVID-19 & Flu A/B At-Home Combo Test
Expiration Date Extension
As of July 1, 2025, the expiration date of the Flowflex Plus COVID-19 and Flu A/B Home Test has been extended by 9 months. Please click here to check the new expiration date using the lot number printed on the test kit box.

Keep Your Family Safe.
Flu A&B/COVID-19 Home Test from
America’s most trusted brand.
Results in 15 minutes.
Easy to Use. Fast Results.
Please refer to the Quick Reference Instructions for detailed instructions
Step 1
Swab
Step 2
Swirl
Step 3
Drop
Step 4
See Results
Easy Step-by-Step Video Tutorials
Frequently Asked Questions

Disclaimer
- In the USA, this product has not been FDA cleared or approved, but has been authorized by FDA under an Emergency Use Authorization.
- This product has been authorized only for the detection of proteins from SARS-CoV-2, influenza A and influenza B, not for any other viruses or pathogens.
- The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. §360bbb- 3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
For more information on EUAs please visit: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization
For the most up to date information on COVID-19, please visit: www.cdc.gov/covid
Leading Technology
For more than two decades, ACON has led the way in making high quality diagnostic and medical devices more affordable to people all around the world.