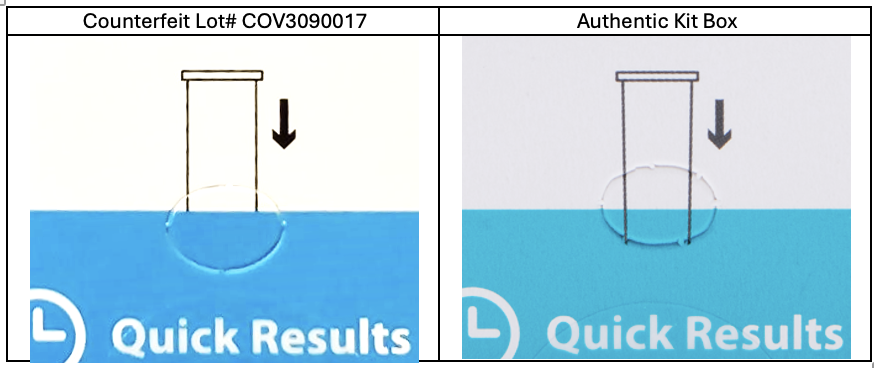
ACON Laboratories, Inc. has become aware of counterfeit Flowflex COVID-19 Antigen Home Test kits displaying Lot number COV3090017 which are being illegally sold in the United States and Puerto Rico by unauthorized distributors. Although these counterfeit test kits appear to display an authentic Flowflex COVID-19 Antigen Home Test Lot number, these counterfeit test kits were not manufactured, imported, or distributed by ACON Laboratories. Counterfeit test kits displaying Lot number COV3090017 may be distinguished by examining the front of the kit box. On the counterfeit COV3090017 kit boxes, the sides of the illustrated tube do not extend into the teal. On authentic kit boxes, the sides of the illustrated tube extend into the teal and stop at the edge of the perforation.
Individuals who have purchased or received Flowflex test kits displaying Lot number COV3090017 and believe that the tests may be counterfeit should save the test kit packaging and any unused tests and contact ACON’s customer support at 1-800-838-9502.
Authentic Flowflex COVID-19 Antigen Home Tests can be purchased from retail chain stores, major online resellers such as Amazon.com, or one of these authorized distributors.
Individuals who encounter counterfeit tests may also report it to the FDA as a voluntary adverse event report at: MedWatch, the FDA’s Safety Information and Adverse Event Reporting Program. Alternatively, problems can be reported to FDA using either method below:
Consumer Reporting Form FDA 3500B. Follow the instructions on the form to either fax or mail it in for submission. For help filling out the form, see MedWatchLearn.
Call FDA at 1-800-FDA-1088 to report by telephone.
If you have any questions or concerns, please contact ACON’s customer support at 1-800-838-9502.