ACON Laboratories, Inc., has become aware that counterfeit versions of its FDA-authorized Flowflex® COVID-19 Antigen Home Tests in the 1 Test/ kit box configuration marked as Lot# COV2010030 and COV2030016 are being illegally distributed in the United States through unauthorized distributors. These counterfeit tests have not been authorized, cleared, or approved by the FDA for distribution or use in the United States, but their packaging and components may very closely resemble authentic FDA-authorized Flowflex tests.
Consumers should take precautions to avoid purchasing Flowflex in the 1 Test/ kit box configuration marked as Lot# COV2010030 and COV2030016, that was not distributed through an authorized distributor. ACON is currently not aware of any counterfeit tests in the 2 Test/ kit box and 5 Test/ kit box configurations. The performance of these counterfeit tests has not been adequately established and there is concern about the risk of inaccurate results when people use these unauthorized tests.
Flowflex tests from Lot# COV2010030 and COV2030016 purchased from ACON’s authorized distributors or retail partners listed on FlowflexCOVID.com are authentic and safe to use.
The counterfeit tests can be identified by the following differences from authentic Flowflex tests:
Lot# COV2010030
- The product name, COVID-19 Antigen Home Test, on the top panel of the counterfeit test kit has a skinny letter “I” in “COVID-19”, whereas the authentic test kit has consistent sizing.

- The Global Trade Item Number, GTIN “(01)” printed on the counterfeit kit box label starts with 20 and ends with 265, whereas the authentic GTIN for the 1 Test kit starts with 00 and ends with 261.

- The cassette pouch inside the counterfeit test kit has bolding in the text and the text is not aligned with the symbols, this is inconsistent with the authentic cassette pouch.

Lot# COV2030016
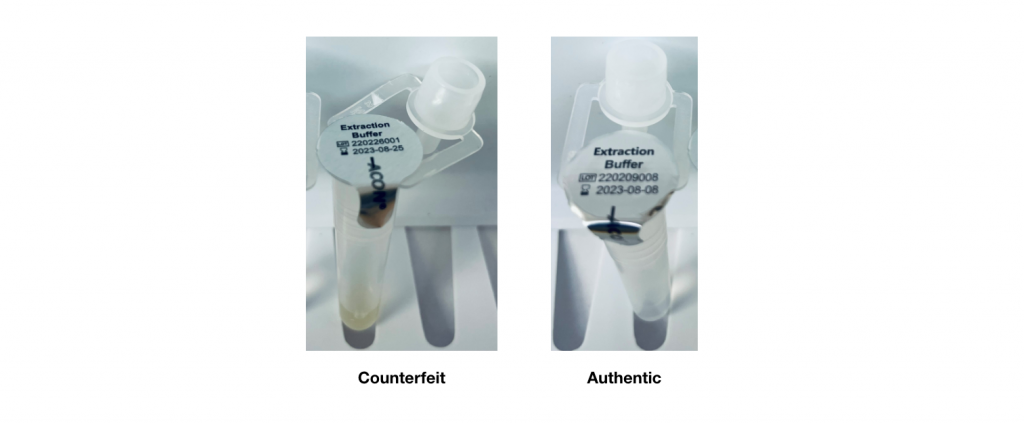
- The counterfeit test kit has an extraction buffer tube with a yellow-colored solution, whereas the authentic test kit has an extraction buffer tube with a clear and colorless solution.

- The tab of the foil seal on the extraction buffer tube inside the counterfeit test kit is not aligned to the dropper tip attached to the tube. The tab of the foil seal on the extraction buffer tube inside the authentic test kit is aligned to the dropper tip.
This is not a recall, as these counterfeit tests were not manufactured, imported, or distributed by ACON Laboratories. Retailers and consumers who encounter or possess counterfeit tests should not use them and should destroy them. ACON Laboratories is unable to receive returns of any such counterfeit tests you may possess.
If you have information regarding the distribution or sale of counterfeit Flowflex tests in the United States, please contact ACON Laboratories at info@aconlabs.com.
Individuals who encounter problems with the counterfeit test can report it to FDA as a voluntary adverse event report at: MedWatch, the FDA’s Safety Information and Adverse Event Reporting Program. Alternatively, problems can be reported to FDA using either method below:
- Consumer Reporting Form FDA 3500B. Follow the instructions on the form to either fax or mail it in for submission. For help filling out the form, see MedWatchLearn.
- Call FDA at 1-800-FDA-1088 to report by telephone.
